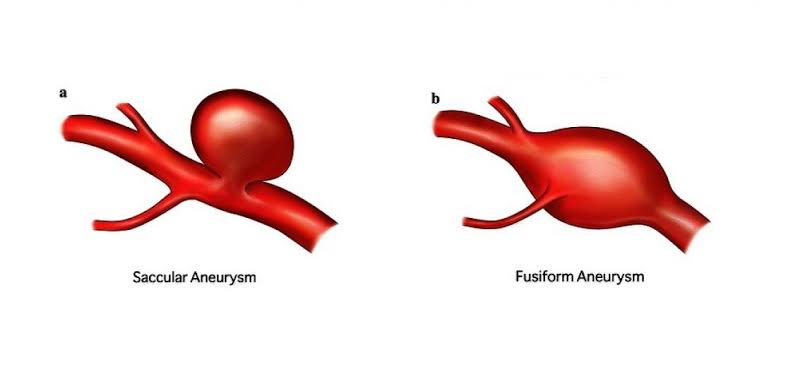
• Also known as: CBC; Hemogram
• Sample Required?
- A blood sample drawn from a vein in your arm or a fingerstick or heelstick (newborns)
• Test Preparation Needed?
- None
• Why get tested?
- To determine your general health status; to screen for, diagnose or monitor any one of a variety of diseases and conditions that affect blood cells, such as anemia, infection, inflammation, bleeding disorder or cancer.
• Also known as: Hgb; Hb; H and H (Hemoglobin and Hematocrit)
• Sample Required?
- A blood sample drawn from a vein in your arm or a fingerstick or heelstick (newborns)
• Test Preparation Needed?
- None
• Why get tested?
- To evaluate the hemoglobin content of your blood as part of a general health check-up; to screen for and help diagnose conditions that affect red blood cells (RBCs); If you have anemia (low hemoglobin) or polycythemia (high hemoglobin), to assess the severity of these conditions and to monitor response to treatment
• When to get tested?
- With a hematocrit or as part of a complete blood count (CBC), which may be ordered as a component of a general health screen; when you have signs and symptoms of anemia (weakness, fatique) or polycythemia (dizziness, headache); at regular intervals to monitor these conditions or response to treatment
• Also known as: Thrombocyte count; PLT; Platelet distribution width; PDW; Mean Platelet volume; MPV.
• Sample Required?
- A blood sample drawn from a vein in your arm or a fingerstick or heelstick (newborns)
• Test Preparation Needed?
- None
• Why get tested?

- To determine the number of platelets in a sample of your blood as part of a health exam; to screen for, diagnose, or monitor conditions that affect the number of platelets, such as a bleeding disorder, a bone marrow disease, or other underlying condition.
• When to get tested?
- As part of a routine complete blood count (CBC); when you have episodes of unexplained or prolonged bleeding or other symptoms that may be due to a platelet disorder
• What is being tested?

- Platelets, also called thrombocytes, are tiny fragments of cells that are essential for normal blood clotting. They are formed from very large cells called megakaryocytes in the bone marrow and are released into the blood to circulate. The platelet count is a test that determines the number of platelets in a person’s sample of blood. When there is an injury to a blood vessel or tissue and bleeding begins, platelets help stop bleeding.
• Also known as: Leukocyte differential count; Peripheral differential; WBC count differential; Diff; blood differential; Differential Blood Count
• Formal name: White blood cell differential
• Why get tested?
- To help determine the cause of abnormal results on a WBC count; to help diagnose or monitor an illness affecting your immune system, such as an infection or inflammatory condition, or cancers that affect your white blood cells, such as leukemia.
• When to get tested?
- As part of a CBC; when you have a routine health examination; when results of a CBC fall outside the reference range; when you have any number of signs and symptoms that may be related to a condition affecting white blood cells, such as infection, inflammation, or cancer, when you are receiving treatment that is known to affect WBCs, such as chemotherapy.
• What is being tested?
- WBCs, also called leukocytes, are cells that circulate in the blood and the lymphatic system that help protect the body against infections. They are an important part of the body’s immune system and also have a role in inflammation, allergic responses, and protection against cancer. A WBC differential totals the number of each of the different types of WBCs in a person’s sample of blood.
- There are five types of white blood cells, each with different functions.
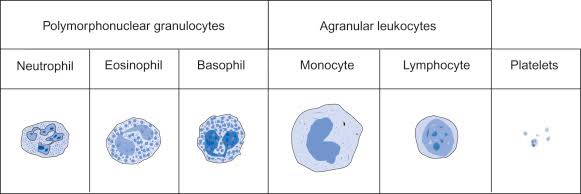

- Also known as: TLC; WBC count
- Total WBC count is used as one of the index of presence of systemic infection and to rule out the possibility of leukemia & malignant neutropenia
- Calculated with haemocytometer/ automated cell counts
- RBCs are lysed by diluting the blood sample with dilute acetic acid leaving the WBCs intact.
- Also known as: Red Blood Cell Count, RBC count
- Red blood cells, also known as erythrocytes, make up the cellular part of blood, giving it its red color and also the ability to bind and carry oxygen to all parts of the body. Under a microscope, they appear to be circular and biconcave in shape.
- Gives us the number of erythrocytes per cubic mm in circulating blood & Hb in blood.
- Procedure done by office or chairside method and also automated procedure.
- Hematological diseases of RBCs are anemia & polycythemia.
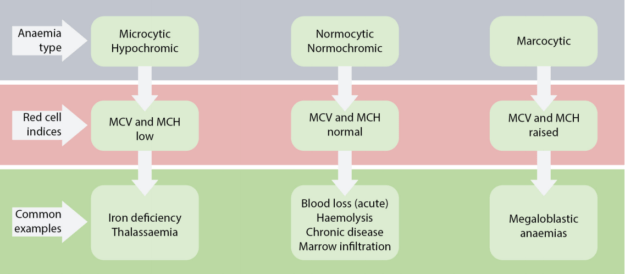
- Categorized by mean corpuscular volume, anemia can be differentiated into microcytic, macrocytic and normocytic anemias. Normocytic anemia can be further divided into intrinsic and extrinsic RBC defect and blood loss.
- MCV – Mean corpuscular volume is the average volume of red blood cells and is reflective of RBC size. When RBCs increase or decrease in size, the mean corpuscular volume changes; this helps physicians determine the type of anemia and its causes. Normal MCV is 80–96 µm³.
- MCH stands for “mean corpuscular hemoglobin.” An MCH value refers to the average quantity of hemoglobin present in a single red blood cell.
- MCHC is short for mean corpuscular hemoglobin concentration. MCHC refers to the average amount of hemoglobin inside a single red blood cell.
- Hematocrit is the measure of the total volume % of red blood cells in the blood. The normal value for hematocrit is 45% for men and 40% for women. It is an important component of a patient’s complete blood profile.
Indications:
- To prepare smears from paper points removed from root canals for evaluation of microcytic status of canal prior to filling.
- A scraping or swab of an oral lesion is needed to confirm diagnosis of thrush
- A scraping of gingival region or mucosal ulcer is sometimes used to confirm diagnosis of Acute Necrotising ulcerative stomatitis.
- Identification of giant cells that accompany vesicular infections
- Identification of Acantholysis
Dentowesome|@drmehnaz🖊
References: Google.com, lecturio.com, Study Notes✍🏻