









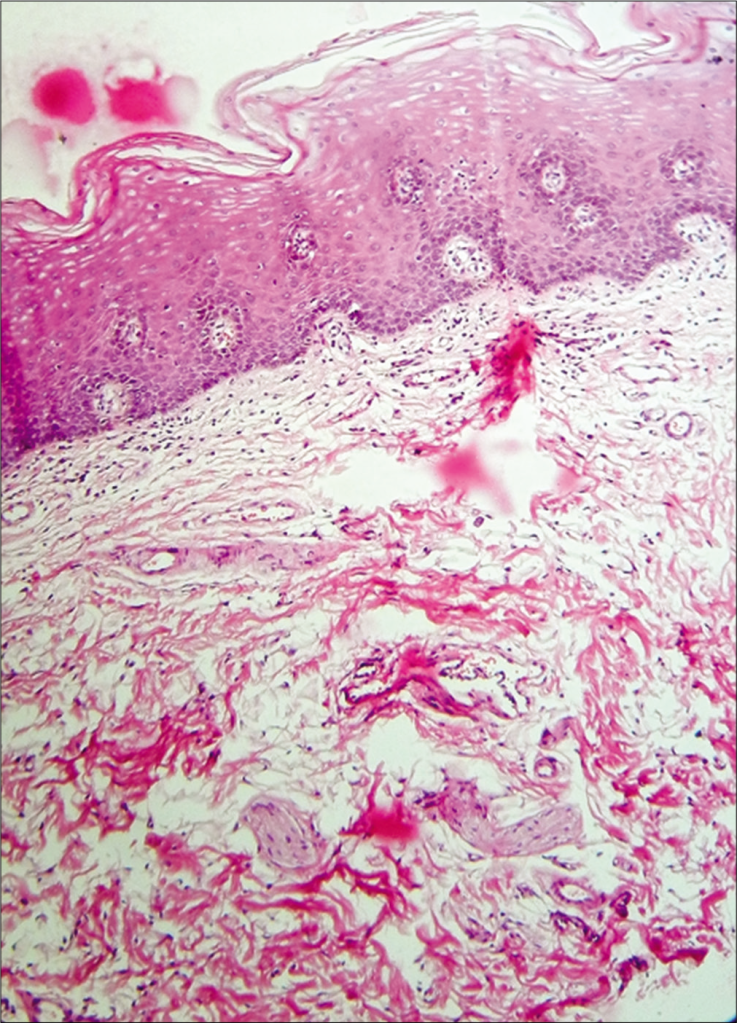
Sections show buccal mucosa in which there is mild epithelial atrophy with parakeratosis. The pattern of epithelial maturation is regular and the overall architecture is preserved. The rete processes are flattened and bands of hyaline collagen best seen in Van Geison stained sections are present in the lamina propria. A mild chronic inflammatory infiltrate is present in the subepithelial tissue.
Our adventure starts with a visit to the microscopic realm of the buccal mucosa – the inner lining of the cheek. Imagine a bustling cityscape with layers of epithelial cells, each playing its role in maintaining the oral harmony. But wait, something’s not quite right here!
🔬 Clue 1: The Atrophy Enigma
The buccal mucosa seems to be undergoing a transformation – a mild epithelial atrophy. It’s as if the cells are shrinking, losing some of their vitality. Parakeratosis is in play too, where these cells are holding onto their nuclei longer than they should. It’s like they’re not quite ready to grow up and shed their immature ways.
📜 Clue 2: The Architectural Anomaly
Despite the changes, the overall architectural blueprint of the buccal mucosa remains intact. The maturation of the epithelial cells follows a regular pattern, almost like well-practised dancers performing a choreographed routine. The rete processes – the finger-like projections that interlock the layers – appear flatter than usual. It’s as if they’re tired and can’t stand as tall as they used to.
🔍 Clue 3: The Mysterious Collagen Chronicles
Ah, now for a fascinating twist! Van Geison stained sections reveal bands of hyaline collagen lurking in the depths of the lamina propria – the supporting layer beneath the epithelium. These collagen bands are like secretive cobwebs, weaving a mysterious tale of their own. Their presence hints at something more profound beneath the surface.
🔥 Clue 4: The Inflammatory Intrigue
As our investigation deepens, we stumble upon an unexpected guest – a mild chronic inflammatory infiltrate. It’s almost like a small group of protesters voicing their concerns beneath the epithelial cityscape. What could they be protesting? What’s causing this subtle turmoil?
🚀 The Grand Reveal: Unveiling Submucous Fibrosis
Now, my fellow detectives, armed with our clues and insights, it’s time for the big reveal! The answer to this intriguing riddle is none other than Submucous Fibrosis.
🕵️♂️ Unraveling the Mystery
Submucous Fibrosis is a condition often linked to the chewing of paan (betel), a common practice in certain cultures. In this condition, dense collagenous bands sneakily weave their way into the lamina propria – that’s the collagen we spotted earlier! These bands tighten their grip, causing limitations in mouth opening and even trouble with swallowing.
But wait, there’s more! The potential consequences get even more serious. With these collagenous infiltrators running amok, there’s a risk of dysplasia – that’s abnormal cell growth – and even the development of oral cancer.
A 65-year-old man went through some serious stuff. 🙌 He had a mandibular rim resection and neck dissection to tackle squamous-cell carcinoma in the floor of his mouth and ventral tongue. 🦠 The pathologist’s notes spill the beans – the tumor was mainly hanging out in the mouth floor, measuring 28mm wide and 11mm deep. And guess what? Out of 48 lymph nodes from the neck dissection, 5 were playing host to some sneaky squamous-cell carcinoma guests, 2 even decided to venture out of their capsules! 🏃♂️💥 So, what’s the verdict?
So, in fancy doctor lingo aka Union for International Cancer Control, they call it pT2 when the tumor’s between 2 and 4cm big, and pN2b when you got a bunch of nodes involved but none are bigger than 6cm. 🤷♂️
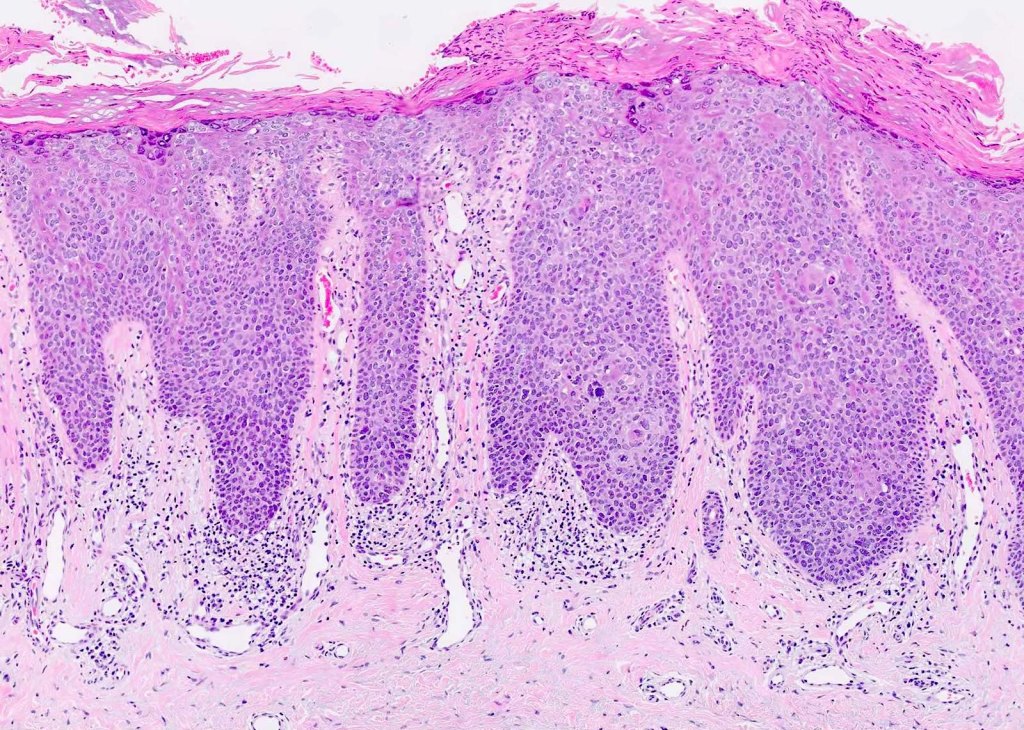
Sections show oral mucosa. In the oral epithelium there is basal-cell crowding and hyperplasia. Atypical mitotic figures are present throughout the thickness of the oral epithelium. The squamous cells show nuclear and cellular pleomorphism, and keratin whorls are present. The rete ridges are drop shaped and individual cell keratinisation is present in some areas.
The diagnosis here is 🔍 Carcinoma in situ! 🦠
In simple terms, it’s like a full-blown drama show happening in the oral epithelium! 🎭 The cells are misbehaving – basal-cell crowding, hyperplasia, and atypical mitotic figures are causing chaos! 🤯🔬
The squamous cells are like divas with nuclear and cellular pleomorphism, and there are even keratin whorls for added glam! 💁♀️✨
The rete ridges are shaped like drops, adding some artistic flair, and individual cell keratinisation is stealing the spotlight! 💅🌟
Now, here’s the twist – severe epithelial dysplasia is often considered a prelude to this drama, and together they’re sometimes known as Squamous Intraepithelial Neoplasia Grade 3 (SIN 3)! 📜🌆
Histological examination shows sheets of squamous cells supported by fibrous stroma. Keratin pearls are present and there is focal necrosis. The squamous cells are pleomorphic and possess hyperchromatic nuclei. Numerous atypical mitotic figures are present. The invasive front is non-cohesive and there is a moderate chronic inflammatory infiltrate at the invasive front.
Hey, curious minds! 🌟 Let’s dive into some histology secrets! 🕵️♀️ Imagine peeping into a microscope and spotting a tissue slide with all the drama – squamous cells forming sheets, keratin pearls, and even focal necrosis! 😱 But that’s not all, the squamous cells look all wild – they’re pleomorphic, with hyperchromatic nuclei and atypical mitotic figures dancing around! 🧪🔬 And guess what? At the front lines, there’s an invasion party going on – non-cohesive and joined by a squad of chronic inflammation! 💥🦠
Hold onto your lab coats, because we’re unveiling the diagnosis – it’s none other than squamous-cell carcinoma! 🦠🔍 This is like a villainous takeover in the world of cells! So, next time you’re in histology class, remember these telltale signs of squamous-cell carcinoma! 📚🩺
RESEARCH – Diagnostic methods and treatment modalities
Squamous-cell carcinoma is a type of oral cancer that can be diagnosed and treated using various options. Diagnostic aids and adjunctive techniques, such as toluidine blue, brush cytology, tissue chemiluminescence, and autofluorescence, can assist in the screening of healthy patients for evidence of cancerous changes or to assess the biologic potential of abnormal mucosal lesions (Lingen et al., 2008). Human papillomavirus (HPV) testing is recommended for oropharyngeal cancer, and treatment options for oropharyngeal squamous-cell carcinoma include radical radiotherapy or transoral surgery and neck dissection (Shah et al., 2016). Additionally, squamous-cell carcinoma of the oral cavity evolves within a field of precancerized oral epithelium, and individuals who have been successfully treated for oral squamous-cell carcinoma are at high risk of developing a recurrence (Feller et al., 2013).
A longstanding tumour was removed from the parotid gland. The pathologist reported that the tumour was composed of sheets, strands and islands of ductal cells separated by myxochondroid tumour. Plasmacytoid myoepithelial cells were present in some areas and islands of squamous cells were also present. The tumour was enclosed by an intact pseudo- capsule formed by compressed fibrous tissue into which tumour pseudopodia extended.
Hey, peeps! Let’s decode a cool case from the world of dentistry! 🦷🔍 So, there was this long-standing tumor hanging out in the parotid gland. 🤨 The pathologist spilled the tea – the tumor had ductal cells in groups, mixed with myxochondroid stuff! 😲 Plus, there were some plasmacytoid myoepithelial cells hanging around, and even squamous cell islands crashed the party! 🕺🏻💃 The tumor had a slick move too – it had a pseudo-capsule made of tight fibrous tissue!
💪 But guess what? This is known as pleomorphic adenoma, aka a “mixed tumor”! 🎉 ‘Cause it’s a mix of ductal cells and myxochondroid stromal elements! 🧪 And those hyaline plasmacytoid myoepithelial cells? They’re like the signature VIPs of this party! 🤘 Stay tuned for more dental mysteries, y’all! 🌟🦷 #PleomorphicAdenoma #MixedTumorMagic [Link]
RECENT ADVANCES IN PLEOMORPHIC ADENOMA
Pleomorphic adenoma is a common tumor of the salivary glands, particularly the major salivary glands (AlAmari et al., 2021). It typically presents in the third to sixth decades of life (AlAmari et al., 2021). Surgical excision is the mainstay of treatment for pleomorphic adenoma, as these tumors can grow to giant sizes if left untreated (AlAmari et al., 2021). However, recurrent pleomorphic adenoma can be a challenge to treat and has variable outcomes (Kanatas et al., 2018). The recommended treatment for recurrent pleomorphic adenoma includes surgical excision, radiation therapy, or a combination of both (Hemavathy et al., 2022). External beam and neutron radiotherapy may be alternative treatments offered to select patients (Hemavathy et al., 2022).
Recent advances in the diagnosis and treatment of pleomorphic adenoma have focused on improving diagnostic accuracy and refining treatment strategies. Modern imaging techniques, such as MRI, allow for the evaluation of the anatomical extent of the tumor and its relationship to surrounding structures, particularly the facial nerve (Poorten et al., 2011). The World Health Organization (WHO) Histological Classification facilitates accurate and consistent diagnosis of pleomorphic adenoma (Poorten et al., 2011).
In terms of grading and prognostication, there has been a shift in understanding that carcinoma ex pleomorphic adenoma is not automatically a high-grade tumor, as traditionally suggested (Seethala, 2009). Grading schemes for salivary gland carcinomas, including adenoid cystic carcinoma and mucoepidermoid carcinoma, have been developed based on various histological features (Seethala, 2009). These grading schemes help predict the prognosis and guide treatment decisions (Seethala, 2009). Adenoid cystic carcinomas are graded based on the pattern of solid components, with solid components portending a worse prognosis (Seethala, 2009). Mucoepidermoid carcinomas are graded in a three-tier fashion based on various features, including cystic component, border, mitoses, anaplasia, and perineural invasion (Seethala, 2009).
Recent research has also focused on the molecular biology of pleomorphic adenoma. Alterations in the PLAG1 gene have been identified in both benign and malignant pleomorphic adenomas (Martins et al., 2005). These gene alterations play a role in the tumorigenesis of pleomorphic adenoma and may provide insights into the morphogenesis of these tumors (Martins et al., 2005).
In terms of treatment outcomes, postoperative radiotherapy has been shown to improve locoregional control in all stages and grades of parotid carcinoma (Poorten et al., 2011). However, systemic treatment for distant failure remains disappointing, although recent progress in molecular biology has suggested the potential for targeted therapy (Poorten et al., 2011). The prognosis of individual patients can be increasingly accurately predicted through multivariate analysis (Poorten et al., 2011).
In conclusion, recent advances in the diagnosis and treatment of pleomorphic adenoma have focused on improving diagnostic accuracy, refining treatment strategies, and understanding the molecular biology of these tumors. Surgical excision remains the mainstay of treatment, but recurrent pleomorphic adenoma can be challenging to manage. Grading schemes have been developed to predict prognosis and guide treatment decisions for salivary gland carcinomas. Further research is needed to explore targeted therapies and improve outcomes for patients with pleomorphic adenoma.
Cutting-edge tech like MRI and WHO Histological Classification are our allies for precise diagnosis and treatment planning! 📊📸 And guess what? The rulebook for grading and predicting outcomes is getting a makeover! 😲 Carcinoma ex pleomorphic adenoma might not be an automatic baddie – it’s all about those histological features! 📝
We’re delving into the molecular secrets of pleomorphic adenoma with the PLAG1 gene stealing the spotlight! 🧬🔬 Targeted therapy might be the game-changer on the horizon! 🌅 So, while surgery remains the star, the future is filled with potential to conquer these challenges and boost patient outcomes! 🚀
References
AlAmari, K., Zahlan, A., Albawardi, E., Dababo, M., Alotaibi, N. (2021). A Case Report Of a Rare Nasopharyngeal Myoepithelial Dominant Pleomorphic Adenoma. International Journal of Surgery Case Reports, (82), 105859. https://doi.org/10.1016/j.ijscr.2021.105859 Hemavathy, K., V, G., Subramani, V., Susruthan, M. (2022). Recurrent Palatal Pleomorphic Adenoma: a Case Report With A Long-term Follow-up. Cureus. https://doi.org/10.7759/cureus.26363 Kanatas, A., Ho, M., Mücke, T. (2018). Current Thinking About the Management Of Recurrent Pleomorphic Adenoma Of The Parotid: A Structured Review. British Journal of Oral and Maxillofacial Surgery, 4(56), 243-248. https://doi.org/10.1016/j.bjoms.2018.01.021 Martins, C., Fonseca, I., Roque, L., Pereira, T., Ribeiro, C., Bullerdiek, J., … & Soares, J. (2005). Plag1 Gene Alterations In Salivary Gland Pleomorphic Adenoma and Carcinoma Ex-pleomorphic Adenoma: A Combined Study Using Chromosome Banding, In Situ Hybridization And Immunocytochemistry. Modern Pathology, 8(18), 1048-1055. https://doi.org/10.1038/modpathol.3800386 Poorten, V., Bradley, P., Takes, R., Rinaldo, A., Woolgar, J., Ferlito, A. (2011). Diagnosis and Management Of Parotid Carcinoma With A Special Focus On Recent Advances In Molecular Biology. Head & Neck, 3(34), 429-440. https://doi.org/10.1002/hed.21706 Seethala, R. (2009). An Update On Grading Of Salivary Gland Carcinomas. Head and Neck Pathology, 1(3), 69-77. https://doi.org/10.1007/s12105-009-0102-9
A 55-year-old woman presented to her dentist with carious cavities that had occurred since her last check-up. The patient had noticed that her mouth was dry and, on examination, her parotid glands were enlarged. At the dental hospital, her consultant performed a needle core biopsy, which was reported as containing confluent sheets of non-caseating granulomas. She was referred on to an ophthalamic specialist who found that she had uveitis on slit-lamp examination.
OMG, check out this jaw-dropping dental case, peeps! 🦷💥 This 55-year-old woman rolls into her dentist’s office with cavities, but wait, there’s more drama! 🤯 She’s got a dry mouth and super-sized parotid glands! 🌵💧 Like, what’s going on? 🤷♀️ So, she lands in the dental hospital, and her biopsy report comes back with a wild twist – non-caseating granulomas! 😱🔍 But it doesn’t stop there! She’s sent to an ophthalmic specialist and guess what they find? Uveitis on the scene! 👀😓
Whoa, here’s some dental knowledge for you, fam! 🦷💡 Ever heard of Heerfordt’s syndrome? It’s a chronic condition called sarcoidosis, where granulomas go on a wild spree! 🌪️😱 It can hit the parotid glands and cause uveitis, like a double whammy! 👀😓 But wait, there’s more! Facial nerve palsy and fever might join the party too! 🤧🌡️ So, next time you spot this dental detective, think Heerfordt’s syndrome! 🔍💙
Heerfordt’s syndrome, also known as Heerfordt-Waldenström syndrome or uveoparotid fever, is a rare subtype of sarcoidosis. It is characterized by the presence of parotid gland enlargement, facial nerve palsy, uveitis, and low-grade fever (Sève et al., 2021; Denny & Fotino, 2013; Kakizaki et al., 2017; Fujiwara et al., 2016). Complete Heerfordt’s syndrome is diagnosed when all four main symptoms are present, while incomplete Heerfordt’s syndrome is diagnosed when two out of the three symptoms (facial nerve palsy, parotid gland enlargement, and anterior uveitis) are detected (Ahmed et al., 2020; Fujiwara et al., 2016).
The most common symptoms of Heerfordt’s syndrome are facial nerve palsy, parotid gland enlargement, and anterior uveitis (Sève et al., 2021; Denny & Fotino, 2013; Kakizaki et al., 2017; Fujiwara et al., 2016). Other possible manifestations of sarcoidosis include cough, dyspnea, chest pain, weight loss, arthralgias, erythema nodosum, and intrathoracic involvement (Denny & Fotino, 2013; Sève et al., 2021). Intrathoracic involvement, characterized by symmetrical bilateral hilar adenopathy and/or diffuse lung micronodules, is the most common extrapulmonary manifestation of sarcoidosis (Denny & Fotino, 2013). Skin lesions, liver or splenic involvement, peripheral and abdominal lymphadenopathy, and peripheral arthritis are also frequent extrapulmonary manifestations (Denny & Fotino, 2013).
Heerfordt’s syndrome is typically self-limiting and resolves within 12 to 36 months, although some cases may be prolonged (Kakizaki et al., 2017).
Heerfordt’s syndrome – It’s a sneaky subtype of sarcoidosis. 👀🤒 The main players are parotid gland enlargement, facial nerve palsy, uveitis, and a low-grade fever! 🔍🌡️ When all four symptoms party together, it’s complete Heerfordt’s syndrome, but even with two out of three, it’s still incomplete! 🤝🏽💫 Other possible signs include cough, chest pain, weight loss, and more! So keep your eyes peeled for this rare gem! 💎✨ It usually clears up within a year or so, but some cases can be trickier! 🕰️💙
A 56-year-old woman complains of a burning sensation affecting her tongue. It is present on a more or less continuous basis and gets worse as the day goes on. Her GP prescribed a mouthwash for her but this has not been of any benefit. She is edentulous but leaving her dentures out makes no difference to the pain. Her medical history is unremarkable and, on examination, her tongue appears completely normal. What is the diagnosis? Click on below link to find out the answer
Hey, guys! 🙋♀️ So, there’s this 56-year-old lady, and she’s like, “My tongue feels like it’s on fire 🔥 all the time, ugh!” Her GP gave her this lame mouthwash, but it’s like, zero help. 🙅♀️ Like, why even bother, right? She doesn’t even have teeth, and guess what? Taking out her dentures doesn’t make a dang difference to the pain! 😬 Her medical history is all chill, and when they checked her tongue, it’s totally normal. 🤷♀️ So, what could be causing this burning sensation? It’s like a mystery, y’all! 🕵️♀️
So, like, if you’re on the same page as me, you’re probably DYING to know the diagnosis, right?! I gotchu covered! Just click on the link below to reveal the big answer! 🕵️♀️
Alright, brace yourselves for the big reveal! 🥁 drumroll, please 🥁 The diagnosis is… (wait for it) … Burning Mouth Syndrome (BMS)! 🙌 Yeah, you heard me right! It’s this condition where you feel this burning sensation in your mouth, but there’s no apparent cause! 😵
I know, I know, it’s like, totally insane! 😜 But BMS is for real, yo! It’s this mysterious thing that can happen to anyone, even if they don’t have teeth! 🦷 It’s like your taste buds are throwing a party, but instead of fun, it’s just burning vibes. 🔥 And it’s not just her, peeps! Lots of people deal with this, and it can be super frustrating! 😤
So, even though her mouth looks fine, she’s stuck with this annoying burning sensation. 🙅♀️ But don’t worry, she’s not alone! There are ways to manage this crazy BMS beast, like staying away from spicy foods or acidic stuff that can make it worse. 😓
RESEARCH
Burning Mouth Syndrome (BMS) is a chronic oral pain disorder characterized by a burning sensation in the mouth without any specific mucosal lesions (Kim et al., 2020). It is more common in women, particularly in the fourth to sixth decades of life (Scardina et al., 2010). The exact cause of BMS is still unknown, but it has been associated with various factors such as psychoneurological conditions, peripheral nerve atrophy in the tongue epithelium, and psychosocial events (Kim et al., 2020).
Several studies have reported an increased prevalence of psychiatric symptoms and psychological disorders in patients with BMS, including depression and anxiety (Kim et al., 2020). In a population-based cohort study conducted in South Korea, it was found that patients with BMS had a higher risk of developing depression and anxiety compared to individuals without BMS (Kim et al., 2020). The adjusted hazard ratios for the development of depression and anxiety were 2.77 and 2.42, respectively (Kim et al., 2020).
In addition to psychiatric symptoms, BMS has also been associated with other chronic pain syndromes, such as other idiopathic orofacial pain and central sensitivity syndromes (Moisset et al., 2016). This suggests that BMS may share common mechanisms with other chronic pain conditions (Moisset et al., 2016).
Treatment options for BMS are limited and there is no gold-standard treatment available (Çinar et al., 2018). However, various approaches have been explored. One study evaluated the efficacy of a topical capsaicin rinse in improving the symptoms of BMS and found it to be useful in treating the discomfort associated with BMS (Silvestre et al., 2012). Another study investigated the effectiveness and safety of clonazepam, pregabalin, and alpha-lipoic acid for treating BMS and found that systemic clonazepam and pregabalin were viable options for treatment (Çinar et al., 2018). Acupuncture has also been suggested as a therapeutic possibility for BMS (Scardina et al., 2010).
In conclusion, BMS is a chronic oral pain disorder characterized by a burning sensation in the mouth. It is associated with an increased risk of developing psychoneurological conditions, such as depression and anxiety. The exact cause of BMS is still unknown, but it has been linked to peripheral nerve atrophy, psychosocial events, and other chronic pain syndromes. Treatment options for BMS are limited, but topical capsaicin, systemic clonazepam, pregabalin, and alpha-lipoic acid have shown promise in improving symptoms. Further research is needed to better understand the etiology and develop more effective treatments for BMS.
REFERENCES
Kim, Y., Ko, I., Kim, D. (2020). Association Between Burning Mouth Syndrome and The Development Of Depression, Anxiety, Dementia, And Parkinson Disease. JAMA Otolaryngol Head Neck Surg, 6(146), 561. https://doi.org/10.1001/jamaoto.2020.0526 Moisset, X., Calbacho, V., Torres-Martínez, P., Gremeau-Richard, C., Dallel, R. (2016). Co-occurrence Of Pain Symptoms and Somatosensory Sensitivity In Burning Mouth Syndrome: A Systematic Review. PLoS ONE, 9(11), e0163449. https://doi.org/10.1371/journal.pone.0163449 Scardina, G., Ruggieri, A., Provenzano, F., Messina, P. (2010). Burning Mouth Syndrome: Is Acupuncture a Therapeutic Possibility?. Br Dent J, 1(209), E2-E2. https://doi.org/10.1038/sj.bdj.2010.582 Silvestre, F., Silvestre-Rangil, J., Tamarit-Santafé, C., Bautista, D. (2012). Application Of a Capsaicin Rinse In The Treatment Of Burning Mouth Syndrome. Med Oral, e1-e4. https://doi.org/10.4317/medoral.17219 Çinar, S., Kartal, D., Pergel, T., Borlu, M. (2018). Effectiveness and Safety Of Clonazepam, Pregabalin, And Alpha Lipoic Acid For The Treatment Of Burning Mouth Syndrome. Erciyes Med J, 35-38. https://doi.org/10.5152/etd.2018.17160
An 11-year-old patient, following trauma to the jaw as an infant, developed a worsening facial asymmetry and now has extreme limitations in opening. He has no other joint problems in the body.
The problem is localised to a single joint in the body and has a clear relationship to trauma when an infant, so ankylosis seems a likely diagnosis and juvenile chronic arthritis seems unlikely. Bony ankylosis means that mandibular movement is essentially non-existent, although a few millimetres of movement may be observed through flexing of the bone. Sometimes, however, the ankylosis is due to fibrous union of the joint components, and a little greater movement may be possible. Radiography should be able to differentiate the two types.
RESEARCH CORNER
Bony ankylosis in an 11-year-old individual can be caused by trauma. Trauma-induced bony ankylosis has been reported in various studies (Rikhotso & Nkonyane, 2017; Dhupar et al., 2018; Gomes et al., 2017).
For example, Rikhotso & Nkonyane (2017) reported a case of bony ankylosis in an 11-year-old resulting from untreated zygomatic arch fracture when the patient was 2 years old (Rikhotso & Nkonyane, 2017).
Similarly, Gomes et al. (2017) stated that trauma is responsible for a significant proportion of cases of ankylosis (Gomes et al., 2017).
Additionally, young trauma patients are more likely to develop more severe types of ankylosis (Xia et al., 2019).
Therefore, the occurrence of bony ankylosis in an 11-year-old individual can be attributed to trauma experienced in infancy.
References
Dhupar, V., Akkara, F., Khandelwal, P., Louis, A. (2018). Zygomatico-coronoid Ankylosis As Sequel Of Inadequate Treatment. Ann Maxillofac Surg, 1(8), 158. https://doi.org/10.4103/ams.ams_107_15 Gomes, A., Pereira, G., Santos, Í., Matos, J., Franco, J., Santos, M., … & Neto, I. (2017).
Ankylosis Due Sequel Of Fracture Of the Mandibular Condyle: Case Report. int arch med, (10). https://doi.org/10.3823/2507 Rikhotso, R., Nkonyane, M. (2017).
Zygomatico-coronoid Ankylosis: a Case Report. OJST, 11(07), 475-480. https://doi.org/10.4236/ojst.2017.711043 Xia, L., An, J., He, Y., Xiao, E., Chen, S., Yan, Y., … & Zhang, Y. (2019).
Association Between the Clinical Features Of And Types Of Temporomandibular Joint Ankylosis Based On A Modified Classification System. Sci Rep, 1(9). https://doi.org/10.1038/s41598-019-46519-8
A 54-year-old woman developed an unsightly cyst on the nape of the neck which oozed cheesy material.
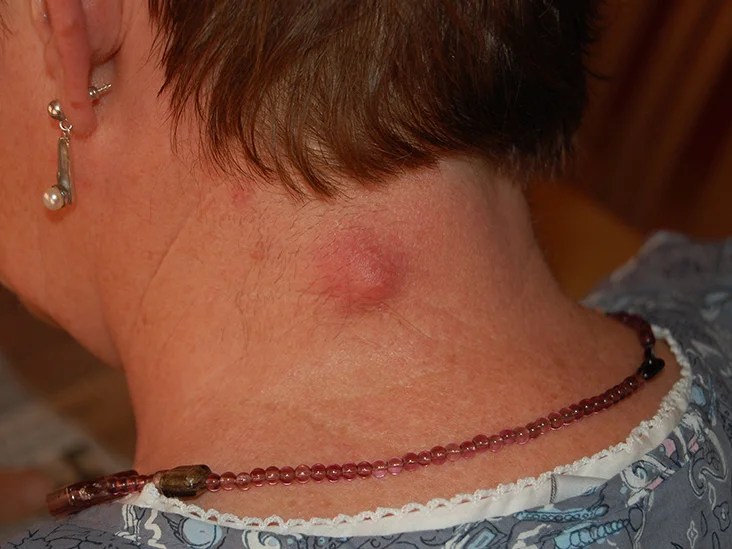
Epidermal cysts are very common and occur anywhere on the head and neck skin and at other sites. They are lined by stratified squamous epithelium and contain oily keratinous material.
Epidermal cysts are common skin lesions that can occur in various parts of the body, including the neck, face, and perineal area (Kang et al., 2011; Pawde & Kathale, 2014; Gupta et al., 2020). They are the most common cysts of the skin (Kang et al., 2011). In some cases, epidermal cysts can be associated with genetic syndromes such as Gardner’s syndrome, which is characterized by extraintestinal manifestations including dental abnormalities (Guilmette & Nosé, 2017). Dental abnormalities, including epidermal cysts, can also be seen in other inherited gastrointestinal cancer syndromes (Achatz et al., 2017). The treatment of choice for epidermal cysts is surgical excision, which is considered to be the first-line effective treatment (Suh et al., 2017; Yu et al., 2021). Epidermal cysts are typically benign, but rare cases of squamous cell carcinoma arising from an epidermal cyst have been reported (Al-Zawi et al., 2019).
References: Achatz, M., Porter, C., Brugières, L., Druker, H., Frebourg, T., Foulkes, W., … & Plon, S. (2017). Cancer Screening Recommendations and Clinical Management Of Inherited Gastrointestinal Cancer Syndromes In Childhood. Clinical Cancer Research, 13(23), e107-e114. https://doi.org/10.1158/1078-0432.ccr-17-0790 Al-Zawi, A., Memon, S., Shah, A., Eldruki, S., Tan, E., Alowami, S. (2019). A Squamous Cell Carcinoma Arising From Scrotal Epidermal Cyst. a Case Report And Review Of 94 Cases From The World Literature. Nowotwory Journal of Oncology, 3-4(69), 150-156. https://doi.org/10.5603/njo.2019.0028 Guilmette, J., Nosé, V. (2017). Hereditary and Familial Thyroid Tumours. Histopathology, 1(72), 70-81. https://doi.org/10.1111/his.13373 Gupta, R., Verma, P., Bansal, N., Semwal, T. (2020). A Case Of Ruptured Perineal Epidermal Cyst. Cureus. https://doi.org/10.7759/cureus.11099 Kang, S., Kim, C., Cho, H., Park, M., Lee, Y., Cho, M. (2011). Two Cases Of Giant Epidermal Cyst Occurring In the Neck. Ann Dermatol, Suppl 1(23), S135. https://doi.org/10.5021/ad.2011.23.s1.s135 Pawde, Y., Kathale, S. (2014). Fine Needle Aspiration Cytology As a Diagnostic Tool In Head And Neck Lesions. jemds, 45(3), 11072-11079. https://doi.org/10.14260/jemds/2014/3445 Suh, K., Kang, D., Park, J., Yang, M., Kim, J., Lee, K., … & Jang, M. (2017). Usefulness Of Dermoscopy In the Differential Diagnosis Of Ruptured And Unruptured Epidermal Cysts. Ann Dermatol, 1(29), 33. https://doi.org/10.5021/ad.2017.29.1.33 Yu, Q., Wang, Y., Shi, Y., Gu, J. (2021). A Rare Case Of Facial Multiple Epidermal Cysts: Successfully Treated By Surgical Excision.. https://doi.org/10.21203/rs.3.rs-892579/v1