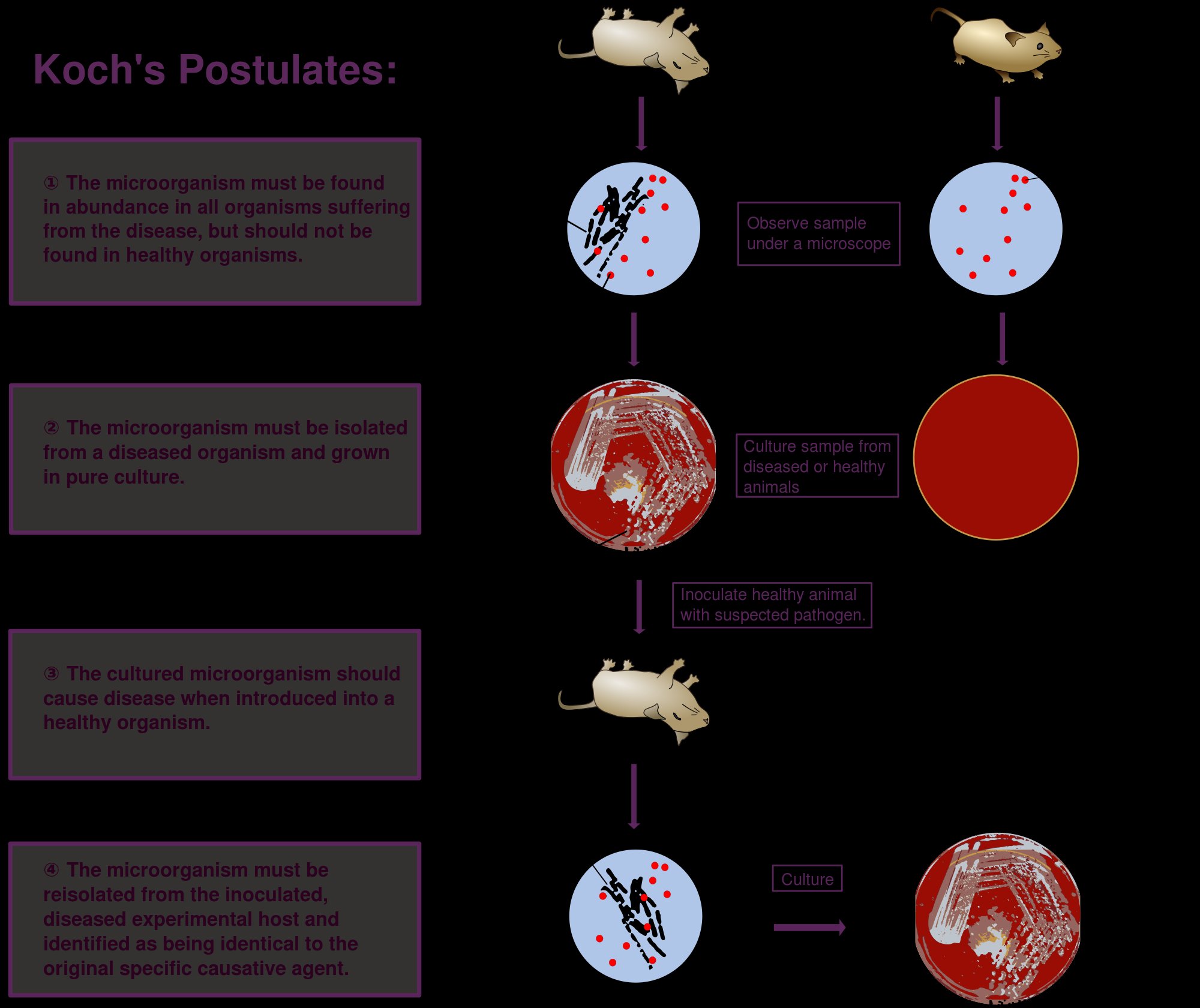
A wide range of viruses may cause liver inflammation (hepatitis) and several
are relevant to dentistry. Here, I describe the recognized family of hepatitis viruses A to G.
Hepatitis A virus (HAV)
The hepatitis A virus is:
- A member of the Enteroviridae.
- It is a spherical, non-enveloped virus.
- It has a single-stranded RNA genome.
- HAV initially replicates in the gut, followed by a viremic phase, during which the virus enters the liver.
Transmission
• Fecal-oral transmission.
• Endemic worldwide.
Diagnosis
• Demonstration of HAV antigen in feces.
• Serology: detection oflgM anti-HAV.
Clinical features
• The incubation period is 2-7 weeks.
Many infections are asymptomatic. Clinical disease is mild with few complications. There is no carrier state.
Prevention and Control
Good hygienic measures and sanitary disposal of excreta.
Passive immunization gives immediate protection for 3-6 months.
Active vaccination: the formalin-inactivated vaccine provides protection for up to 10 years.
HAV is not a major cross-infection hazard in dentistry but is a hazard if traveling, especially to the tropics.
Hepatitis B virus (HBV)
This highly infectious blood-borne virus poses a major cross-infection hazard
in surgery and dentistry:
• It is a member of the hepadnavirus family.
• The intact viral particle (Dane particle) has a double-shelled structure, with the outer hepatitis B surface antigen (HBsAg) coat surrounding the central hepatitis B core antigen (HBcAg), DNA, and DNA polymerase.
• Peripheral blood of infected patients also contains non-infective spherical and filamentous particles of HBV
Transmission
• HBV can be present in blood, saliva, cervical secretions, and semen.
• Spread is via the parenteral route, especially by intravenous drug use, but transmission by intimate contact and sexual activity also occur.
• Perinatal infection is important in certain parts of the world, for example east and southeast Asia.
• There is a large reservoir of unidentified carriers within the population.
• Infected patients may have up to 1010 Dane particles per ml of blood; as little as 0.0001 ml of blood may transmit the infection.
• HBV has been transmitted in dentistry, to patients and dental staff. Some have died from infection.
Diagnosis
• Serological.
• Initial screening is for HBsAg; if present, it indicates infection with HBV.
• Screen then for HBeAg. If present, the person is at high risk for transmission.
• A minority who are HBeAg negative can also transmit infection. Hepatitis B carriers produce HBsAg and, in high-risk carriers, HBeAg for many years.
• Development of anti-HBs, anti-Hbe, and anti-HBc antibodies is associated with recovery. The incubation period is 2-3 months duration. There are a number of possible outcomes of exposure to HBV:
• Subclinical infection (65%).
• Acute hepatitis B with full recovery (30%).
• Chronic carriage (up to 9% of adults): this gives a long-term risk of cirrhosis, liver failure, and hepatocellular carcinoma. Carriers remain infectious to others.
• Fatal fulminant hepatitis (1 %).
Prevention and Control
• Modifications to behavior.
• Adequate infection control procedures in clinical practice.
• Passive immunization: hyperimmune hepatitis B immunoglobulin is used following a single acute exposure in an unprotected individual.
• Active immunization. Hepatitis B vaccine consisys of20 mg ofHBsAg given intramuscularly at 0, l, and 6 months. Boosters have been recommended at 5-year intervals. All vaccinees should have their serum antibody level assessed after vaccination. High-risk carriage of HBV should be excluded in non-responders who are health care workers.
• Interferon may be effective in the treatment of chronic HBV infection.
Hepatitis C virus (HCV)
This blood-borne virus, discovered in 1989, is responsible for most cases of what was previously known as parenterally transmitted non-A, non-B hepatitis(NANBH). Is an enveloped RNA virus.
• Is related to animal pestiviruses and human flaviviruses.
• Has multiple genotypes.
• Cannot be grown in tissue culture.
Diagnosis
• Serological.
• Initial detection of HCV antibodies.
• Confirmation by PCR for HCV RNA.
Transmission
• The prevalence of HCV antibodies among UK blood donors is 0.1-0.3%.
• In recipients of blood products and among intravenous drug users the seroprevalence is high(> 80%).
• Parenteral transmission is the major route, especially in intravenous drug use.
• Sexual transmission is inefficient.
• Occupational transmission may be through needlestick injuries, though it is less infectious than HBV.
• Undefined routes: in a significant number ofHCV-infected individuals, the route of infection is unknown.
Clinical features
• The mean incubation period is 6-12 weeks.
• Acute disease is mild and often subclinical.
• Chronic disease is common (> 60%). These patients may develop longterm liver disease, including hepatocellular carcinoma. Some patients may develop oral disorders similar to Sjogren’s syndrome or lichen planus.
Prevention and Control
• Changes in behavior, e.g. needle exchange schemes for intravenous drug users.
• Screening of donated blood.
• Effective universal infection control in health care settings.
• No vaccine is available.
• Treatment of chronic carriers with interferon and ribavirin is effective in
about 40% of cases.
Hepatitis D virus (HDV)
• A defective, independently transmissible agent which requires hepatitis B virus for replication.
• In developed countries it is mainly a problem among intravenous drug users.
• The genome is single-stranded RNA.
• Transmission is primarily parenteral, either at the time of first infection with HBV (co-infection) or during a subsequent exposure in a patient already infected with HBV (superinfection).
• HDV increases the severity of HBV infection and fulminant hepatitis is common.
• HDV has been transmitted in dentistry, to patients and dental staff. Dental patients have died from infection.
Hepatitis B vaccination is protective.
Hepatitis E virus
This recently discovered virus causes the disease described previously as enterically transmitted non-A, non-B hepatitis:
• It is a spherical, non-enveloped, RNA virus.
• Transmission is via fecal!y contaminated drinking water.
• The incubation period is 2-9 weeks.
• It mainly affects young adults.
• Infection is usually self-limiting.
• There are no chronic carriers.
• Infection carries a high mortality (up to 20%) in pregnancy.
• It is not a major cross-infection risk in surgery.
Hepatitis G virus
• Hepatitis G is a flavivirus, first isolated in 1995 from a patient with chronic hepatitis.
• Seroprevalence studies show evidence of infection in 3% of blood donors in the United Kingdom, 18% of hemophiliacs, and 33% of intravenous drug users.
• Hepatic damage appears mild or absent and the virus is not considered an important pathogen.
• It is not a major cross-infection risk in surgery.
Dr Iswarya V
General Practitioner,
Trivandrum.
Reference : Oxford Clinical Dentistry